Overview: AI-guided closed-loop experimentation has recently emerged as a promising method for materials discovery. Achieving the full potential of this approach in the chemical sciences requires new and efficient methods to access large chemical spaces. In our group, we use AI-guided methods to discover new functional materials for applications including organic electronics and light-harvesting materials. Candidate molecules suggested by AI/ML frameworks are prepared using a modular, ‘Lego-like’ molecular building block approach based on Suzuki cross-coupling, which enables access to large chemical spaces.
Automated Synthesis for Molecular Electronics
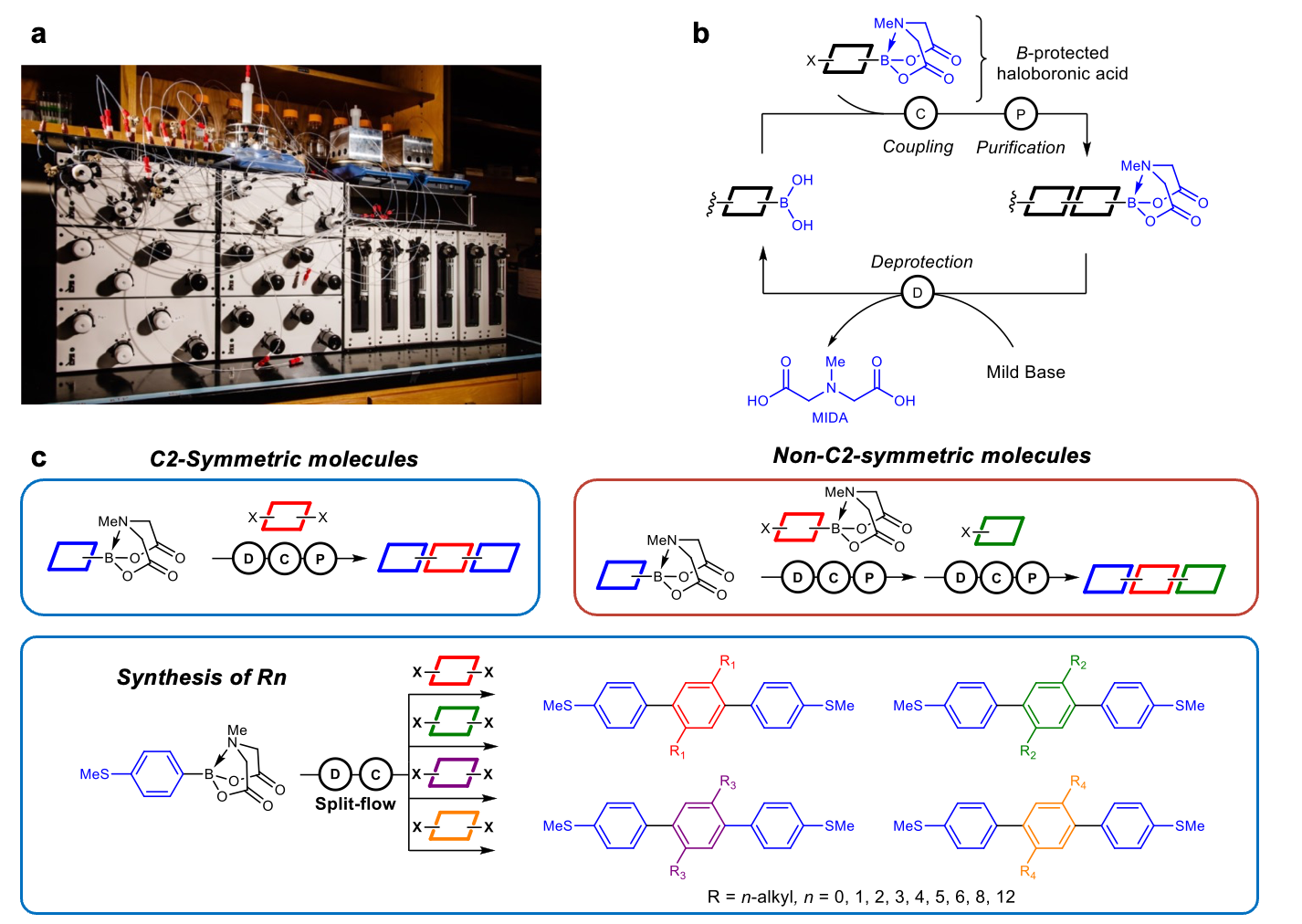
The development of next-generation organic electronic materials critically relies on understanding structure-function relationships in conjugated polymers. However, unlocking the full potential of organic materials requires access to their vast chemical space while efficiently managing the large synthetic workload to survey new materials. In recent work, we used automated modular synthesis to prepare a library of conjugated oligomers with systematically varied side chain composition followed by single-molecule characterization of charge transport. Our results show that molecular backbones with long alkyl side chains exhibit a bimodal conductance with an unexpectedly high conductance state that arises due to surface adsorption and backbone planarization, supported by a series of control experiments using asymmetric, planarized, and sterically hindered molecules. DFT simulations and control experiments highlight the role of side chain chemistry on charge transport. Overall, this work opens new avenues for using automated synthesis for the development and understanding of organic electronic materials.
Publication:
S. Li, E. R. Jira, N. H. Angello, J. Li, H. Yu, J. S. Moore, Y. Diao, M. D. Burke, C. M. Schroeder, “Using Automated Synthesis to Understand the Role of Side Chains on Molecular Charge Transport”, Nature Communications, 13, 2102 (2022).
Closed-Loop Optimization of Reaction Conditions
General conditions for organic reactions are important but rare, and efforts to identify them usually consider only narrow regions of chemical space. Discovering more general reaction conditions requires considering vast regions of chemical space derived from a large matrix of substrates crossed with a high-dimensional matrix of reaction conditions, rendering exhaustive experimentation impractical. In recent work, we used a simple closed-loop workflow that leverages data-guided matrix down-selection, uncertainty-minimizing machine learning, and robotic experimentation to discover general reaction conditions. Application to heteroaryl Suzuki-Miyaura cross-coupling identified conditions that double the average yield relative to a widely used benchmark that was previously developed using traditional approaches. This study provides a practical road map for solving multidimensional chemical optimization problems with large search spaces.

Publication:
N. H. Angello, V. Rathore, W. Beker, A. Wolos, E. R. Jira, R. Roszak, T. C. Wu, C. M. Schroeder, A. Aspuru-Guzik, B. A. Grzybowski, M. D. Burke, “Closed-loop Optimization of General Reaction Conditions”, Science, 378, 399-405 (2022).