Supercharged protein assembly
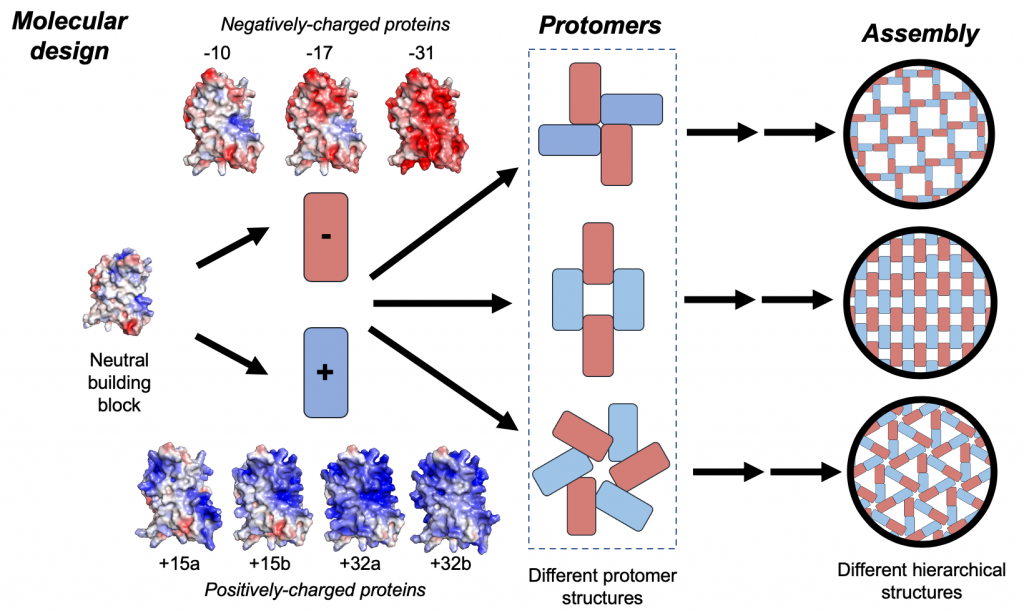
The assembly of biologically encoded molecules into ordered supramolecular structures gives rise to unique properties in natural biological systems. Ordered supramolecular assemblies of supercharged synthetic proteins have recently been created using electrostatic interactions between oppositely charged proteins. Despite recent progress, the fundamental mechanisms governing the assembly process between oppositely supercharged proteins are not fully understood. In recent work, we used a combination of experiments and computational modeling to systematically study the supramolecular assembly process for a series of oppositely supercharged green fluorescent protein (GFP) variants. Our results show that the assembled structures of oppositely supercharged proteins critically depend on surface charge distributions. In addition, net charge is a sufficient molecular descriptor to predict the interaction fate of oppositely charged proteins under a given set of solution conditions (e.g., ionic strength). Interestingly, our results show that a large excess of charge is necessary to nucleate assembly and that charged residues that are not directly involved in interprotein interactions contribute to a substantial fraction (~30%) of the interaction energy between oppositely charged proteins via long-range electrostatic interactions. Dynamic subunit exchange experiments enabled by Förster resonance energy transfer (FRET) further show that relatively small, 16-subunit assemblies of oppositely charged proteins have kinetic lifetimes on the order of ~10-40 minutes, which is governed by protein composition and solution conditions. Overall, our work shows that a balance between kinetic stability and electrostatic charge ultimately determine the fate of supramolecular assemblies of supercharged proteins.
Selected publications:
- M. I. Jacobs, P. Bansal, D. Shukla, C. M. Schroeder, “Understanding Supramolecular Assembly of Supercharged Proteins”, ACS Central Science, 8, 1350–1361 (2022).
Pi-conjugated peptides
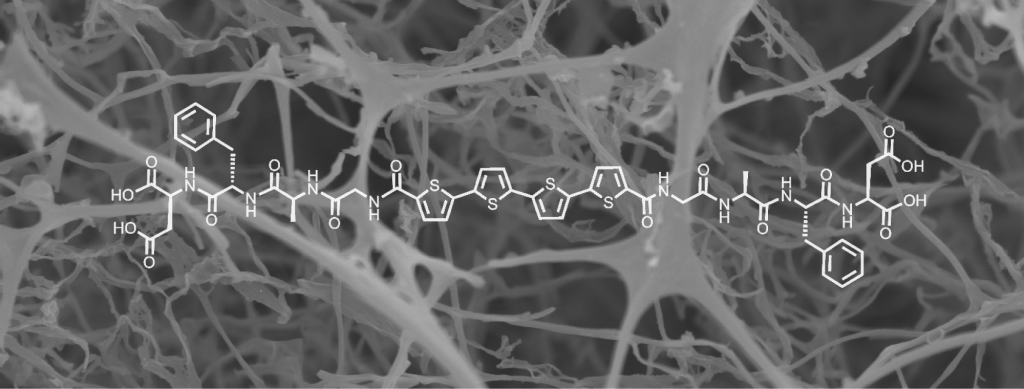
In nature, biomolecules such as proteins and DNA exhibit precise control over chemical sequence, giving rise to well-defined structure and function. On the other hand, synthetic organic molecules offer a near limitless chemical diversity at the cost of full sequence control. Hybrid materials contain both natural biological components and non-natural components, thereby combining the advantages of biomaterials and synthetic materials. We study the functional properties of hybrid bio-synthetic materials, with one example consisting of natural peptides linked to pi-conjugated organic molecules. These materials provide access to electronically active, biocompatible materials with defined supramolecular structures. We are exploring the vast chemical space to design and understand the properties of next-generation, self-assembling electronic materials.
Selected publications:
- E. R. Jira, K. Shmilovich, T. S. Kale, A. Ferguson, J. D. Tovar, C. M. Schroeder, “Effect of Core Oligomer Length on the Phase Behavior and Assembly of pi-conjugated Peptides”, ACS Applied Materials & Interfaces, 12, 20722 (2020).
- L. Valverde, B. Li, C. M. Schroeder, W. Wilson, “In Situ Photophysical Characterization of pi-conjugated Oligopeptides Assembled via Continuous Flow Processing”, Langmuir, 35, 10947 (2019).
- Y. Zhou, B. Li, S. Li, H. A. M. Ardoena, W. L. Wilson, J. D. Tovar, C. M. Schroeder, “Concentration-Driven Assembly and Sol-Gel Transition pi-Conjugated Oligopeptides”, ACS Central Science, 3, 986 (2017).
- B. Li, L. R. Valverde, F. Zhang, Y. Zhou, S. Li, Y. Diao, W. L. Wilson, C. M. Schroeder, “Macroscopic Alignment and Assembly of pi-conjugated Oligopeptides using Colloidal Microchannels”, ACS Applied Materials & Interfaces, 9, 41586 (2017).
- A. B. Marciel, M. Tanyeri, B. D. Wall, J. D. Tovar, C. M. Schroeder*, W. L. Wilson*, “Fluidic-directed Assembly of Aligned Oligopeptides with pi-conjugated Cores, Advanced Materials, 25, 6398 (2013).